
EDGE by Revance
Partnership Program
Terms & Conditions
Updated January 23, 2026
EDGE by Revance Partnership Program Rules and Eligibility
The EDGE by Revance Partnership Program (the "Program" or "EDGE"), is a program owned and operated by Revance Therapeutics, Inc. ("Revance," "we," "us," or "our"), and its affiliates, including any parent or subsidiary companies, or any other companies under common control, and is available and provided to you ("account," "you" or "user") under the terms and conditions set forth herein, including any amendments thereto and with any additional terms and conditions, rules or policies that may be applicable to particular offerings made in connection with the Program (collectively, the "Terms and Conditions") and all applicable laws and regulations.
The Program applies to your eligible purchases of Revance products DAXXIFY® and/or RHA® Collection by Teoxane of dermal fillers and/or SkinPen®, MicroPen EVO™, and/or BIOJUVE® and/or Votesse® (collectively, the “Products”). Eligibility for the Program is determined by the dollar amount of Product purchases based on PARTNER level pricing, less any returns. The timing and total spend of purchases (excludes taxes, shipping, returns, and no-charge products) are determined based on the date of Product order. Only Products directly invoiced by Revance and purchased through its authorized distributor(s) will count toward the Program, including Program benefits.
Your failure to follow the Program's Terms and Conditions or rules, whether set forth below or in supplemental notices posted at various points in the Program, may result in termination of your access and participation in the Program and all benefits you have accrued in the Program, without notice, in addition to Revance's other remedies.
This Program supersedes and replaces all other healthcare professional reward programs applicable to the Products. These Terms and Conditions supersede all previous loyalty program rules and/or terms and conditions applicable to the Products.
These Terms and Conditions may be modified by Revance from time to time, without advanced notice. Any such changes shall be reflected in an updated version of the Terms and Conditions posted at revanceaesthetics.com/edge-terms.
BY PARTICIPATING IN THE PROGRAM, YOU ACCEPT, WITHOUT LIMITATION OR QUALIFICATION, ALL OF THE TERMS AND CONDITIONS. You agree that Revance will not be liable to you or any third party for any modification or discontinuance of the Program, in whole or in part. All references in these Terms and Conditions to a “semi-annual” shall mean a calendar six-month period (January 1 - June 30 and July 1-December 31).
EDGE by Revance Partnership Program Benefits
EDGE provides certain Program benefits, as described below, based on Product purchases. EDGE consists of five (5) levels based on the semi-annual spend for Products, as described in the table below. You must purchase the minimum dollar amount of Products in each semi-annual period, as set forth in the chart below, in order to become eligible at that level and maintain your eligibility at that level for corresponding Program benefits. Initial and Special Tier Qualification for 2026: Upon the launch of the EDGE by Revance Partnership Program on February 1, 2026, the initial tier qualification will be determined based on the cumulative total of qualifying purchases of Revance products from December 1, 2025 – January 31, 2026. This initial evaluation will set the level for each account. From February 1, 2026, your level, corresponding savings discount, and other Program benefits for a semi-annual period will be determined based on the cumulative qualifying dollar amount purchases of Products based on PARTNER level pricing that you made during the previous semi-annual period. The semi-annual periods shall be January 1 – June 30, and July 1 – December 31. The qualifying Product total spend of purchases (excludes taxes, shipping, returns, and no-charge products) and corresponding Pricing benefits are described in the chart below.
For net new accounts, the initial tier qualification will have a pending status until the first qualifying product purchase is made. Upon placement of that order and the calculation of its total, qualifying spend, an EDGE Level will be assigned to the account. Your account’s level and corresponding savings discount are eligible for an instant upgrade at the time of order if the qualifying order dollar amount, combined with previous qualifying purchases in that semi-annual period, reaches the dollar amount threshold for that level. The highest level achieved, and the associated savings discount will be applied for the rest of that semi-annual period and the following semi-annual period. Your level may also be downgraded in the event that any approved returns result in qualifying purchases amount threshold no longer being reached for a certain level. For example, if a purchase brings your account’s, qualifying semester total to or above $15,000, your level would progress from PARTNER to ADVOCATE upon time of purchase, but if returns are approved for $500, your new level will be PARTNER.
Beginning February 1, 2026, qualifying purchases of Products are eligible for Pricing benefits based on your Program level, as provided in the table below.
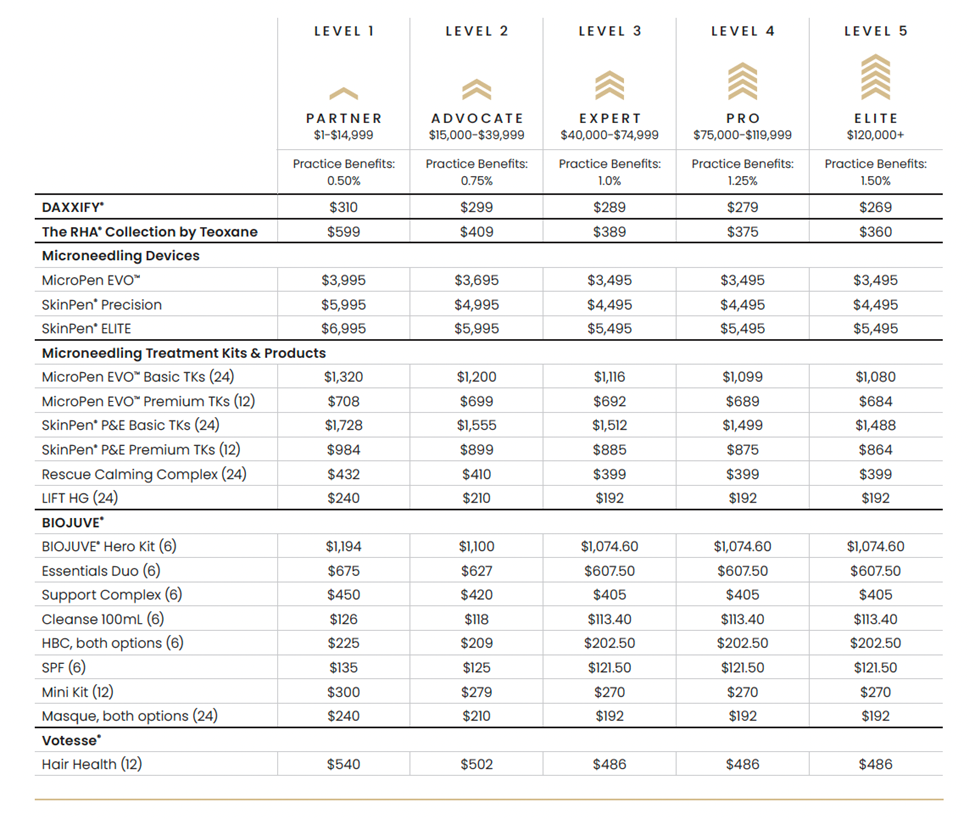
Minimum Order Quantities of 10 vials for DAXXIFY and 5 boxes for RHA Collection by Teoxane still apply, no matter the tier.
The EDGE Credit benefits described above are awarded and exercisable in real-time following the period in which they are earned, as determined by the participant’s cumulative qualifying purchase of products during the applicable current and prior periods. All benefits awarded shall remain valid for a minimum duration of six months and a maximum duration of twelve months from the date of issuance.
For example, EDGE Credits earned February 1, 2026, through June 30, 2026, will be eligible for redemption through December 31, 2026. On January 1, 2027, the Edge Credits earned from February 1, 2026, through June 30, 2026, will no longer be available. EDGE Credits earned from July 1, 2026, through December 31, 2026, will not expire until July 1, 2027.
Revance may, from time to time, add additional benefits to the EDGE by Revance Partnership Program on a temporary or permanent basis.
PROGRAM STATUS
Your Program status and ordering information can be accessed through the EDGE by Revance Partnership Program website, available February 3, 2026, or via an assigned sales representative, if applicable, and via email (support@revance.com) or phone (877-3REV-NOW).
Program Limitations
This Program is limited to licensed healthcare providers in the contingent United States of America, Alaska, Hawaii, and U.S. Territories who purchase participating/qualifying Revance products from Revance through its authorized distributor(s). You may not resell the product to other healthcare professionals without the prior written consent of Revance. Any or all rewards, savings, discounts, rebates, or benefits under this Program may not be transferred to any other party, and each reward, savings, discount, rebate, or benefit under this Program shall be considered void if obtained fraudulently or where prohibited or restricted by law. The Program is void where prohibited or restricted by law. Revance reserves the right to limit purchase quantities.
Benefits described in the program are not redeemable for cash, transferable, or assignable for any reason and cannot be sold, traded, bartered, auctioned through an online auction site, or otherwise; any such Program benefits may be confiscated by Revance and/or canceled.
Reservation of Rights
The Program and its benefits are offered at Revance's discretion, and Revance has the right to modify or discontinue, temporarily or permanently, the Program, in whole or in part, for any reason, at our sole discretion. You agree that Revance will not be liable to you or any third party for any modification or discontinuance of the Program.
Use of Your Information and Personal Information
The information you provide in connection with the Program will be used by Revance and its affiliates, agents, contractors, and vendors for the administration of the Program and to provide you with information about the Program, Program benefits, and Revance Aesthetics Portfolio Products. Revance may also use any information you provide for our internal purposes, including to better understand your needs and how to improve our processes, products and services, and to send you information about Revance and its products and services, as well as special offers or other opportunities from Revance or its business partners that may be of interest.
Revance understands that protecting your personal information (such as name, address, telephone number, email address, and other information) is very important. Revance does not share any personal information you provide us with third parties for their own marketing use.
By participating in the Program, you agree to Revance sharing your information, including your personal information, with third parties including Revance’s affiliates, contractors, agents, and vendors to better provide Revance and Revance affiliated products and/or services. Revance may also combine your personal information with information from third parties to better match special offers with your interests. If you do not wish Revance to use or share your information or send information to you about the Program, Revance, and/or Revance’s products and services, and special offers, you should not participate in the Program. By participating in the Program, you agree we may collect, share and use any information about you that you provide us in accordance with these Terms and Conditions and our Privacy Policy which can be found at https://www.revance.com/privacy-policy/.
Limitation of Liability; Release
Revance and its affiliates and their respective representatives, agents, directors, officers, shareholders, and employees (“Revance Entities”) are not responsible for and shall not be liable for: (i) telephone, electronic, hardware or software program, network, Internet, computer or other malfunctions, failures, or difficulties of any kind, whether human or technical; (ii) failed, incomplete, garbled, or delayed computer transmissions; (iii) any condition caused by events beyond our control; (iv) any injuries, losses, or damages of any kind arising in connection with or as a result of a benefit or acceptance, delivery or failure to timely deliver, possession, or use of a benefit, or from participation in the Program; or (v) any printing or typographical errors in any materials associated with the Program. Further, in no event shall the Revance Entities be liable for any damages of any kind or nature, including but not limited to, direct, indirect, incidental, consequential, exemplary, special (including loss of revenue or profit), punitive, or other damages arising from or in connection with the existence or use of the Program, or any such dispute, regardless of whether any of the Revance Entities have been advised as to the possibility of such damages. You acknowledge that you are solely and exclusively responsible for accurately reporting prices, income, taxes or other information to government agencies and other entities, as applicable.
Proprietary Rights
All Revance and Product names, logos, and service marks in these Terms and Conditions are registered or unregistered trademarks owned by or licensed to Revance or our affiliates, unless otherwise identified as being owned by another entity. Nothing contained herein shall be construed as conferring by implication, estoppel, or otherwise any license or right, either express or implied, under any patent or Trademark of Revance or any third party. No use of any trademark may be made without our prior written authorization.
Governing Law & Disputes
These Terms and Conditions shall be governed by and construed in accordance with the laws of the State of Tennessee without regard to choice of law principles. All applicable federal, state, and local laws and regulations apply. The invalidity or unenforceability of any provisions of these Terms and Conditions shall not affect the validity or enforceability of any other provision. In the event that any provision of these Terms and Conditions is found to be invalid or unenforceable, these Terms and Conditions shall be construed in accordance with their terms as if the invalid or unenforceable provision was not contained therein.
For full Prescribing Information including BOXED WARNING for DAXXIFY®
visit https://hcp.daxxify.com/ . For Directions for Use for the RHA® Collection by Teoxane, visit https://rha.revanceaesthetics.com/. To view SkinPen® intended use, important safety information, and
clinical trial details (data on file), contact us at info@skinpen.com.
©2025 Revance Therapeutics, Inc. All rights reserved. DAXXIFY® is a registered
trademark of Revance Therapeutics, Inc. RHA ® and RHA Redensity® are registered
trademarks of TEOXANE SA, manufactured in Switzerland. The RHA® Collection is
exclusively distributed by Revance. All other trademarks are the property of
their respective owners.
SkinPen®, MicroPen EVO™, BIOJUVE®, and Votesse ® are owned by Crown Aesthetics,
a Revance company.
All products under consumer skincare portfolio are owned by Crown Laboratories,
Inc, a Revance company.